Invasion of the Body Scanners: Innovations in Biomedical Engineering
From: University of Michigan | By: Matthew O'Donnell EDITOR'S INTRODUCTION | Developments in ultrasonic microscopy may soon provide noninvasive solutions to the treatment of medical problems. In this interview with Fathom, Matthew O'Donnell (right), the Jerry W. and Carol L. Levin Professor of Engineering at the University of Michigan, discusses the rise of the biomedical engineering discipline, the relationship between engineering and the medical profession, and the advances his laboratory has made in the creation of biosensing scanners and in medical imaging. In particular, O'Donnell talks about the development of a handheld ultrasonic body scanner to help identify blood clots in postsurgical patients and another device that will improve techniques used in LASIK eye surgery.
EDITOR'S INTRODUCTION | Developments in ultrasonic microscopy may soon provide noninvasive solutions to the treatment of medical problems. In this interview with Fathom, Matthew O'Donnell (right), the Jerry W. and Carol L. Levin Professor of Engineering at the University of Michigan, discusses the rise of the biomedical engineering discipline, the relationship between engineering and the medical profession, and the advances his laboratory has made in the creation of biosensing scanners and in medical imaging. In particular, O'Donnell talks about the development of a handheld ultrasonic body scanner to help identify blood clots in postsurgical patients and another device that will improve techniques used in LASIK eye surgery.Fathom: The field of biomedical engineering is relatively young. What can you tell us about its development and the forces that drive it?
O'Donnell: Biomedical engineering has been around for about forty years at different universities. In the early days, it applied traditional mechanical, electrical, chemical and operations engineering to a specific problem in medicine. These programs were essentially "medical engineering," where a technology would be needed to solve a problem.
The first projects started at the University of Michigan were ventilators, which were used in open-heart surgery. The surgeons who invented the original ventilators did not really understand the equipment that well, and there was a need for engineers to come in, understand and optimize the equipment. The start, then, of biomedical engineering in the 1960s was the application of traditional engineering for problems in medicine — to cure medical problems in a clinical setting.
Fathom: How would you characterize the shift from "medical engineering" to "biomedical engineering"?
O'Donnell: The field started to change in the mid-1980s and early 1990s, when it started becoming clear that scientists were seeking to identify the human genome, and that medical or biomedical research was fundamentally shifting toward microbiology. Many began to rethink what engineering's role would be in all of this and the discipline began to change, dramatically so in the last decade.
The tools that were being developed to study microbiology opened up a suite of applications where engineers could start to think about design. This evolution has led us to realize that biology must be added to the engineer's tool set. Biomedical engineering is the only engineering discipline that uses all of the natural sciences — biology, chemistry, physics and math — to do what engineers do, which is design, optimize and produce real processes and devices.
Fathom: How does the development process work in biomedical engineering? Is it born out of independent research that might impact medical practice, or does it aim to solve a specific problem that exists in medicine?
O'Donnell: Our ultimate customer, we think, is in medicine predominantly — biomedicine. We have an aerospace department at the University of Michigan and the primary industry it serves is aircraft and rocket manufacture. I see us serving the biomedical industry in the same way it serves aerospace. Those engineers do not go out to Boeing and say, "What's your latest problem and that's what we'll work on." No. They research new ideas, new means of combustion, new means of flight control and then see if there are applications for it. Conversely, we also do go out to the companies and see what is pressing and what kind of problems can be solved. We do both.
That is exactly what we are seeing in biomedical engineering. If we ever lose our connection to clinical medicine, we will fail, because these are our ultimate customers, and engineers should be connected to them.
Fathom: Your laboratory's specific focus is on medical imaging and noninvasive biosensing, with applications in diagnostics and treatment. Would you expand on what you do?
O'Donnell: I always use the word "biosense" because our goal is to sense some biological process that is happening using noninvasive means. This means a doctor will not have to stick a probe into a patient to figure out that his blood velocity is half of what is normal, for example. She will be able to do this from outside the body so it will be completely noninvasive. If you can measure a process noninvasively, you can create images of these measurements and patch it together, like a dot puzzle kids follow to make a picture of something.
Our lab at the University of Michigan, which Charles Cain and I have been running for a dozen years, is focused on noninvasive sensing, noninvasive diagnostics and noninvasive therapeutics. The idea here is that if you can sense and localize what is going on in a particular region of the body as being aberrant, then you can try to develop therapeutic systems that will treat it.
For example, if a physician can measure elasticity — the levels of softness or hardness within the body — he might locate microcalcification in the breast, an early sign of breast cancer. Then, knowing its exact location, the doctor may be able to treat the calcification simultaneously using the same type of technology.
Our ultimate goal is to look at molecular processes. We are moving toward imaging not necessarily one molecular event, but a collection of a single type of molecular event, which signifies that a particular process is turned off. A tumor cell, for example, may be identifiable because it may have a receptor that binds a certain molecule. We want to sense this characteristic — a collection of this particular molecule — to identify tumor cells. Once we have identified these collections of cells, we want to kill them specifically, perhaps using lasers. This is therapeutics at the molecular level.
Fathom: This sounds remarkably like the handheld body scanner used by doctors on Star Trek.
O'Donnell: It is funny how science fiction tends to paint reality better than the predictions of the scientist. But I do not think such a scanner is inconceivable. We just keep making steps in that direction.
Fathom: You have actually created such a device to detect thrombosis — blood clots — in the body. Would you talk about that project?
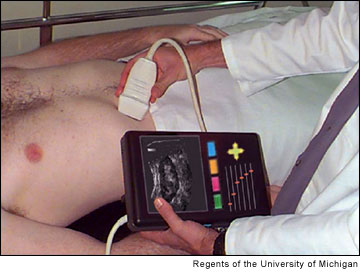
Hand-held ultrasonic body scanner. O'Donnell: We are always trying to tie a process to some parameter that you can see. Deep venous thrombosis, or DVT, is a condition that is the largest cause of hospital death in the country. It results in more deaths per year than breast cancer in this country. It is very common in people who have sedentary lifestyles or are in sedentary circumstances, as those found in postsurgical patients in a hospital setting.
When blood pools, meaning that it does not flow and is static in a vein, it initiates clotting factors, which promote clot formation in a particularly deep vein. This is a big medical problem. A piece of that clot can break off, go to a patient's heart, be pumped up into his or her lungs, and become what is called a pulmonary embolism. Blocking the blood flow can give you either a massive cardiac failure or massive pulmonary failure.
The physician wants to be able to do several things. Number one is to find out if there is a clot in the vein. There are actually pretty well established technologies that allow you to do that; both an MRI (magnetic resonance imaging) and an ultrasound can tell whether there is a clot. The problem here is that while this technology can identify the blood clot, it cannot tell you the age of the clot.
Why does it matter? If this is a very new clot, the patient is at a very severe risk of it breaking off and becoming an embolism. If it's a very old or a chronic clot, the vein has already adjusted to it, or "passivated" it. The older clot is very stable, not going to break out, and so the physician will leave it alone.
To the physician, knowing whether a clot is acute or chronic will radically change the treatment. If it is a new or acute clot, anticoagulants such as Heparin are prescribed. This treatment is very aggressive but can also be very risky; if a patient cuts herself, she can bleed to death.
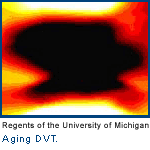 To identify the age of the clot, we looked at the physical parameters. We looked for markers that correlate with the age of the clot. One of the things we learned is very simple: the older the clot, the harder it is. When a clot is very new, it is soft. A fully chronic clot, something that has been in the body for six months or longer, feels as hard as a tough piece of plastic. When we saw this literature and read about these characteristics of clots, we said, "What if you made a noninvasive biosensing of how hard or soft the thing is?" That is what we came up with.
To identify the age of the clot, we looked at the physical parameters. We looked for markers that correlate with the age of the clot. One of the things we learned is very simple: the older the clot, the harder it is. When a clot is very new, it is soft. A fully chronic clot, something that has been in the body for six months or longer, feels as hard as a tough piece of plastic. When we saw this literature and read about these characteristics of clots, we said, "What if you made a noninvasive biosensing of how hard or soft the thing is?" That is what we came up with.
It turns out conventional ultrasound imaging is by far the best method to detect the age of clots because it is simple, cheap and can travel easily to the patient's bedside. With it, we can quantitatively tell you the exact hardness of the clot. And because the hardness correlates with the age of the clot, we can tell you the its precise age, using a little handheld ultrasound transistor.
Fathom: The imaging technology that your lab uses for biosensing includes both magnetic (MRI) and ultrasonic readings. What other ways are there to sense and create medical images?
O'Donnell: An old adviser of mine used to say, "heat, sound, light, electric field, magnetic field — those are your senses." These are the ways that you can see inside of something. Any time that you can monitor change — the way that heat, sound, light, electric field, magnetic field interacts with the body — you can take that information backwards then to figure out what is going on locally.
Our latest approach uses a combination of ultrafast optics and very high frequency ultrasound — in other words, a mixture of light and sound. Thinking about our goal to identify molecular processes, we aim to localize an event so that when we bring in light to it, it creates sound that we can detect. We know that this binding event has occurred because the only way you can hear the sound is if the light was absorbed by this particular structure.
Fathom: When you talk about using light and sound, are you referring to a level of detection that is very sensitive?
O'Donnell: Yes, incredibly sensitive. It is something that you could not perceive with your eye or with your ear.
Fathom: How far off are engineers from being able to detect processes at the molecular level in a medically useable way?
O'Donnell: I think there are some simple examples in development, which are on five- to seven-year timelines. Certain forms of cancer will be detectable much earlier with some of these molecular techniques. Along therapeutic lines, in five to seven years we may be able to very effectively kill a particular well-defined tumor and know that no cells from it remain. That is one of the biggest issues always that comes up in cancer surgery. Now, you have to surgically cut out an area that is much larger than the tumor to hope, not know, but to hope, that all the malignant cells are taken out with it. I think in the five- to seven-year time frame there will be therapies for such a situation with a well-defined tumor, so that we will know that all the cancer cells are dead.
Fathom: One of the other projects you have in development involves fine-tuning LASIK surgery to the individual patient. How does this advancement work?
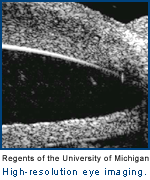 O'Donnell: The whole idea of LASIK surgery is that your eye is a compound lens system. The eye has two lenses in it: one is called the lens, which is inside, and the other is the surface lens, the cornea, and is your primary lens. So in the eye, the outside structure of the cornea is the primary focusing member, and then inside, your lens is a secondary one, which is really the fine adjustment. Glasses correct for the imperfections and curvature that we have in our cornea, for near-sightedness or far-sightedness, for example. Cataracts are a problem with the internal lens.
O'Donnell: The whole idea of LASIK surgery is that your eye is a compound lens system. The eye has two lenses in it: one is called the lens, which is inside, and the other is the surface lens, the cornea, and is your primary lens. So in the eye, the outside structure of the cornea is the primary focusing member, and then inside, your lens is a secondary one, which is really the fine adjustment. Glasses correct for the imperfections and curvature that we have in our cornea, for near-sightedness or far-sightedness, for example. Cataracts are a problem with the internal lens.
Instead of having the patient wear glasses to correct the curvature, LASIK surgery uses a laser to remove tissue from the cornea. Most studies show that this is reasonably effective and very safe. The problem is when the eye changes (as with age), you have to keep taking off more tissue. You cannot put tissue back; you can only take tissue off. The curvature is affected by the thickness and toughness of the cornea and this curvature can be measured by the interocular pressure. When you go to the ophthalmologist, he may take a measuring and say your interocular pressure is twenty millimeters of mercury. There is a fluid in your eye, and it is under pressure; that forces an outward curvature of your cornea. How much the cornea bends is determined by how hard and thick it is.
 During LASIK surgery, the ophthalmologist takes off some of the corneal tissue so it will bend more and be more flexible in a particular way under the same pressure. The problem is the doctor has no clue whether the elasticity of the individual patient's cornea differs from the average value of human cornea elasticity.
During LASIK surgery, the ophthalmologist takes off some of the corneal tissue so it will bend more and be more flexible in a particular way under the same pressure. The problem is the doctor has no clue whether the elasticity of the individual patient's cornea differs from the average value of human cornea elasticity.
An average value works on average very well; however, individuals are unique and so there are many variables. The percentage is low where the LASIK correction is not right, and in those cases it is because the elasticity of the cornea was quite different than what was the assumed normal.
As we age, corneal elasticity changes, and so the correction will also change. In both cases, whether there was a mistake in what was assumed for the original elasticity or because the eye changes with time and aging, the only way you can correct this is to remove more tissue. Eventually the doctor will reach a limit where she cannot take off any more tissue or the cornea will collapse.
 What we have done is to create a way to measure the precise elasticity of the individual patient's cornea, within a few microns of tissue — a small fraction of the width of a human hair. This tool can tell exactly how much tissue to remove and when to stop, and can tell exactly where the curvature is going to be. We can literally guide tissue removal on the level of individual biological cell by individual biological cell. It is perfect for an individual, and because you are minimizing the amount you take off, you can repeat the procedure multiple times as you age and the eye changes.
What we have done is to create a way to measure the precise elasticity of the individual patient's cornea, within a few microns of tissue — a small fraction of the width of a human hair. This tool can tell exactly how much tissue to remove and when to stop, and can tell exactly where the curvature is going to be. We can literally guide tissue removal on the level of individual biological cell by individual biological cell. It is perfect for an individual, and because you are minimizing the amount you take off, you can repeat the procedure multiple times as you age and the eye changes.
Fathom: Does this tool you have developed use ultrasound to measure corneal elasticity?
O'Donnell: Yes, the ultrasound provides the imaging and then the therapeutic piece or surgical piece utilizes ultrafast optics — optical surgery with an ultrasound measurement. Literally, we watch how the image moves when you remove a little tissue: we see how the picture changes.
Fathom: Is this tool readily available today?
O'Donnell: No, it is not on the market. This technology will hopefully be available to ophthalmologists and patients in roughly five years.
Relevant link
University of Michigan Biomedical Engineering(http://www.bme.umich.edu/)