This web page is part of the Michigan Today Archive. To see this story in its original context, click here.
July 9, 2007 Bacteria suggest new approach to Alzheimer's therapy
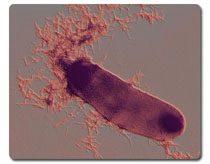
ANN ARBOR, Mich.—New insights into how bacteria form fibers called curli offer intriguing clues to the formation of harmful protein tangles in diseases such as Alzheimer's, Huntington's and Parkinson's, University of Michigan researchers report. Their results will be published online in the Proceedings of the National Academy of Sciences during the week of July 9-13. The research builds on a chance discovery that U-M microbiologist Matthew Chapman and co-workers made five years ago. In research initially aimed at understanding urinary tract infections, they discovered that the common bacterium Escherichia coli makes and employs amyloid fibers, the same types of fibers that are the calling cards of many neurodegenerative diseases. Until then, amyloids were considered "biological blunders" that occurred only when proteins misfolded into deviant forms that aggregate into harmful clumps, Chapman said. But his work showed that bacteria produce amyloid fibers "by design" and use them to adhere to surfaces and to interact with other bacteria. Since making the discovery, Chapman and his lab group have been exploring bacterial amyloids, using an approach that blends microscopy, biochemistry and genetics. In the current work, the researchers reveal details of how curli—functional amyloid fibers assembled by E. coli and certain other bacteria—are assembled. In both bacteria and humans, amyloids form through a process known as nucleation, in which protein subunits link together in a coordinated fashion. Just as a snowflake begins as a speck of dust around which water freezes, an amyloid fiber also requires a template or nucleus to begin forming. In bacteria, two proteins—CsgA and CsgB—are involved in the process, each with its own precise function. The job of CsgA is to build up amyloid fibers, but only after CsgB—dubbed "the nucleator"—has set the stage. "What we've discovered is the molecular mechanism of bacterial amyloid nucleation," said graduate student Neal Hammer, who is lead author on the paper. "The B protein presents an amyloid-like template to the A protein, which builds on that template to form a fiber." Having one protein in charge of nucleation and the other in charge of fiber elongation is a clever strategy that allows for control of a process that otherwise might occur unpredictably, as seems to happen with disease-associated amyloids. "Control is achieved by keeping the A and B proteins apart until they get to the cell surface," said Chapman, an assistant professor in the Department of Molecular, Cellular and Developmental Biology. "At the cell surface, they come together, resulting in controlled amyloid formation." Because CsgB speeds the amyloid fiber formation process, it prevents the buildup of potentially toxic intermediates, Chapman said. Similarly, studies of functional amyloids in other organisms have found that the fibers always form rapidly, bypassing intermediate steps. Such observations suggest new approaches to treating and preventing diseases such as Alzheimer's. "Conventional wisdom has been that if we can prevent fiber formation, we can prevent these diseases," Chapman said. "But if you think about what nature is telling us, it's the exact opposite. I think what these functional amyloids are telling us is that maybe fiber formation is a process that should be happening, and that problems arise when the process goes too slowly and favors these toxic intermediates. Maybe what we should be doing is forcing the protein to form fibers in ways that skip the toxic intermediate steps." Sue Wickner, an investigator at the National Institutes of Health working in the field of protein folding, said, "The Chapman group has been carrying out exciting work that provides novel insight into how amyloid fibers are made. The research has important implications toward a better understanding of the devastating human diseases involving aberrant protein folding, such as Alzheimer's, Creutzfeld-Jacob and dialysis-related amyloidosis." In addition to Chapman and Hammer, Jens Schmidt, a visiting diploma student from Germany, is a co-author on the paper. The researchers received funding from the National Institutes of Health and the Michigan Alzheimer's Disease Research Center.
Related Links: Proceedings of the National Academy of Sciences
Contact: Nancy Ross-Flanigan Related Categories: Health |
|
Michigan Today is a monthly publication for alumni and friends of U-M. |
| MToday NewsE | |
|
|
Michigan Today
online alumni magazine
University Record
faculty & staff newspaper
MGoBlue
athletics
News Service
U-M news
Photo Services
U-M photography
University of Michigan
gateway